Background
The U.S. Food and Drug Administration (FDA), under the Department of Health and Human Services (HHS), safeguards public health by regulating the safety and quality of food, medical products, and more. The Office of Inspections and Investigations (OII) leads all FDA field inspection, investigation, import, emergency response, and enforcement activities, generating critical data that supports regulatory decisions.
For years, these operations relied on more than 50 disconnected systems. Investigators used outdated tools that lacked features like document upload and mobile access. Maintaining these systems required significant resources, while data silos limited visibility and hindered real time collaboration across offices. There was no integration with official document storage nor a consistent way to manage field activities.
In response, the FDA partnered with REI Systems to develop the Regulatory Operations Management System (ROMS), a cloud-native platform built on Appian and integrated with AWS Textract and MuleSoft. ROMS unifies field operations into a secure system supporting Domestic Field Exams, Sample Collections, and Recall Audit Checks (RACs), with real-time data sharing, document management, and enhanced usability for field staff and leadership.
Challenge
FDA’s legacy infrastructure hindered operations, reduced efficiency, and introduced risk by limiting investigators’ ability to perform core field tasks. They lacked access to essential features like document upload, subsample entry, and firm-specific tracking, impacting inspection quality and timely communication.
The systems offered no embedded access to guidance materials like the Investigations Operations Manual (IOM) or Compliance Program Manuals (CPM), leaving users without quick reference to protocols during active fieldwork. Without mobile capabilities or consistent user support, operational delays and manual workarounds became the norm.
Processes like RACs were handled through emailed PDFs and static trackers, making it difficult to verify task completion or escalate findings. The lack of automation and real time insight increased the chance of errors and prolonged regulatory response timelines. Previous modernization efforts often stalled due to limited stakeholder engagement and a narrow focus led by isolated technology teams. Real progress required a business-driven approach and coordinated collaboration across Offices and Divisions. With a strong history of mission-focused, collaborative delivery, Team REI was well positioned to succeed where others had not.
Solutions
Cloud Native Application
 REI Systems delivered ROMS as a cloud native application using the Appian low code platform. The solution, hosted in a secure FDA environment, integrates with AWS Textract, a machine learning service that extracts text from scanned documents, and MuleSoft for real-time data sharing. This architecture supports cloud scalability and on-premise requirements while seamlessly integrating with FDA systems like the Field Accomplishments and Compliance Tracking System (FACTS), the Automated Lab Information System (ALIS), and the Online Reporting Analysis Decision Support System (ORADSS).
REI Systems delivered ROMS as a cloud native application using the Appian low code platform. The solution, hosted in a secure FDA environment, integrates with AWS Textract, a machine learning service that extracts text from scanned documents, and MuleSoft for real-time data sharing. This architecture supports cloud scalability and on-premise requirements while seamlessly integrating with FDA systems like the Field Accomplishments and Compliance Tracking System (FACTS), the Automated Lab Information System (ALIS), and the Online Reporting Analysis Decision Support System (ORADSS).
ROMS also connects with Documentum to ensure regulatory documents are securely stored and centrally accessible. This integration prevents duplication and ensures consistency across the regulatory process, providing a unified and resilient foundation for field operations. It also eliminates the need for manual uploads and scattered document storage that previously slowed collaboration.
Human-Centered Design
ROMS was designed together with FDA users to ensure it met real-world needs from day one. REI collaborated closely with OII leadership and field staff to gather input on business needs, pain points, and user expectations. Stakeholders participated in high-fidelity prototype testing, early feedback sessions, and scenario-based user testing. These activities offered insights into how users naturally navigated the application.
REI expanded beyond traditional user acceptance testing by introducing roadshows, one-on-one reviews, and real-time collaboration across offices. Instead of rigid step-by-step scripts, test scenarios were grounded in real business use cases, prompting more meaningful and actionable feedback. This input directly shaped key ROMS capabilities, including enhanced reporting and field exam functionality. The engagement approach helped surface longstanding pain points, such as the inability to easily find protocol guidance or enter detailed inspection metadata in disconnected systems.
Streamlined Workflows
 The Atomic Regulatory Process—a seven-step model—serves as the foundation for ROMS, streamlining and standardizing field operations across inspections, investigations, and sample collections.
The Atomic Regulatory Process—a seven-step model—serves as the foundation for ROMS, streamlining and standardizing field operations across inspections, investigations, and sample collections.
ROMS currently supports Domestic Field Exams, Domestic Sample Collections, and RACs through dynamic forms, automated logic, and real-time tracking. Investigators can create ad hoc sample collections and exams when connected to the internet, with expanded mobile capabilities for both online and offline use planned for release in September 2025. RACs workflows now include dynamic form validation and autogenerated endorsement types, helping reduce review time and strengthen compliance. Built on this standardized framework, ROMS enables reusability and extensibility for additional operations. The system also provides real-time data sharing, document management, and improved usability for both field staff and leadership—laying a foundation for continued evolution across mission areas.
Training and Adoption
To support implementation, REI and FDA established a proactive engagement model. During the initial phase, key stakeholders met three times per week to align efforts across OII, the Office of Business Informatics and Solutions Management (OBISM), and the Office of Information Management and Technology (OIMT).
 While OII led the initiative, participation from other Offices and Centers ensured a broad range of perspectives. This inclusive approach fostered shared ownership and generated valuable feedback that informed both the initial launch and ongoing development. The FDA’s shift to ROMS marked a deliberate move toward mission-centered digital transformation, characterized by cross functional leadership, iterative delivery, and a long-term plan for usability and growth. OBISM played a key role in guiding delivery based on business needs, while OIMT ensured alignment with the FDA’s enterprise architecture and modernization roadmap.
While OII led the initiative, participation from other Offices and Centers ensured a broad range of perspectives. This inclusive approach fostered shared ownership and generated valuable feedback that informed both the initial launch and ongoing development. The FDA’s shift to ROMS marked a deliberate move toward mission-centered digital transformation, characterized by cross functional leadership, iterative delivery, and a long-term plan for usability and growth. OBISM played a key role in guiding delivery based on business needs, while OIMT ensured alignment with the FDA’s enterprise architecture and modernization roadmap.
The platform moved from concept to production in under 12 months, highlighting REI’s ability to deliver on an accelerated timeline.
Past efforts to modernize similar systems had struggled to gain traction due to siloed leadership and the absence of business input, making this collaboration model a critical success factor.
Features not included in the initial release—such as offline mobile capability—were documented, prioritized, and incorporated into future planning. This transparent process built trust and reinforced confidence in ROMS’s long-term roadmap.
Results
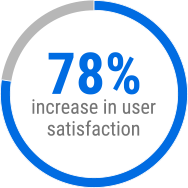
Since implementation, ROMS has significantly improved OII operations. Post launch surveys showed a 78 percent increase in user satisfaction and a 69 percent improvement in system performance. Users reported a 183 percent increase in perceived technical value, reflecting how the new platform supports their work.


ROMS now supports over 5,000 active users and processes more than 6,500 inspection-related transactions each month. Investigators complete tasks more efficiently, while supervisors benefit from improved visibility into timelines, workloads, and outcomes. The centralized platform enables users to assign tasks, conduct field activities, and manage regulatory follow-up—all with integrated document upload and access capabilities.
By automating formerly manual processes, ROMS has improved data quality and reduced investigator burden. Reports are faster to generate, actions easier to track, and operations more efficient. Users no longer need to rely on emailed PDFs, shared trackers, or outdated data entry forms to complete core inspection work. This enables quicker responses to high-risk scenarios and ensures consistent execution of field activities across the country.
Regulatory Impact of ROMS Modernization
ROMS strengthens the FDA’s ability to fulfill its public health mission. Accurate, real-time data and a unified regulatory process allow the agency to act faster, identify emerging risks, and make informed decisions.
 Through integration with FDA’s broader enterprise systems, the platform accelerates data exchange and reduces duplication. Investigators, recall coordinators, and analysts now operate on a modern solution that improves accessibility and ensures greater consistency across the agency. Tools such as MuleSoft APIs and AWS Textract also create opportunities for future automation and continued modernization.
Through integration with FDA’s broader enterprise systems, the platform accelerates data exchange and reduces duplication. Investigators, recall coordinators, and analysts now operate on a modern solution that improves accessibility and ensures greater consistency across the agency. Tools such as MuleSoft APIs and AWS Textract also create opportunities for future automation and continued modernization.
REI’s modernization approach prioritized people, processes, and technology. By aligning ROMS with FDA’s enterprise architecture and establishing continuous feedback loops, REI delivered a solution that is scalable, secure, and built for long-term adaptability. The project serves as a model for mission-aligned modernization, developed in close partnership with end users to ensure lasting impact and operational relevance.
With ROMS, the FDA now operates on a faster and more reliable platform that strengthens field operations and protects product safety. The collaborative delivery model engaged OIMT, OBISM, and field users throughout the process, ensuring enterprise alignment and reducing barriers to adoption. The success of this effort reflects the power of strategic alignment, user-centered design, and a long-term commitment to mission driven modernization, delivering lasting value to the agency and the public it serves.




