CHALLENGE
The Food and Drug Administration (FDA) is charged with making sure food, drugs, medical devices, cosmetics, and other products are safe and effective. One of the many offices within this regulatory body is the Office of Regulatory Affairs (ORA), consisting of, among other offices, the Office of Import Operations (OIO) which keeps a watchful eye on imported products and ensures they comply with regulations.

Amid the ever-evolving landscape of imported products, the Operational & Administrative System for Import Support (OASIS) plays a vital role in supporting the Import Operations business and ensuring a safe and compliant product environment. This system processes and determines the admissibility of FDA-regulated products entering the U.S. and its territories.
However, because it was built on legacy technology, OASIS has struggled to keep pace with the increasing demand and is in dire need of modernization.
SOLUTION

FDA field staff at various international mail facilities nationwide use the application to collect a portion of data electronically with technologies like Optical Character Recognition (OCR) and barcode scanning, even offline. When connected, the data is securely sent to the application servers.
Using Angular technology, SERIO provides a modern, scalable web and mobile app with a single team of developers. The design benefits the FDA, as it needs less technical expertise and has lower maintenance costs, making the application scalable, reliable, and secure.

- SERIO Web uses Angular and a responsive design for a dynamic web interface for investigative, compliance, and international mail facility functions.
- iOS SERIO Mobility is a native iOS app built using NativeScript and Angular to provide essential functions for field operations. It can be used offline when there is no network connection, which is a significant time-saving feature for FDA field staff who used to take notes on paper.
- SERIO uses an Application Programming Interface (API) framework enabling seamless integration and communication with other Imports systems thereby introducing efficiencies (time-savings as well as providing a better user experience).
SOLUTION
The SERIO mobile application has significantly improved the daily work of investigators, inspectors, and warehouse workers constantly on the move. Instead of dealing with manual data entry and paper notes, they can now collect data instantly, leading to fewer mistakes and better-quality notes. Tasks can be completed more efficiently, with the ability to capture photos and record sample information and inspectional observations directly on the mobile device leading to more accurate and complete data.

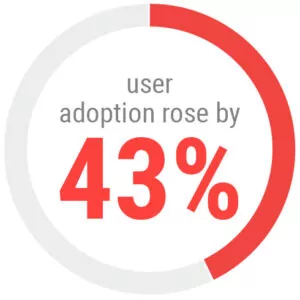
In measurable terms, SERIO’s implementation greatly improved ORA Investigations Branch field officers’ performance. By integrating SERIO with other ORA systems, user adoption rose by an impressive 43%. Moreover, SERIO helped enable a 10% uptick in the number of products examined and a 9% average rise in compliance actions taken.

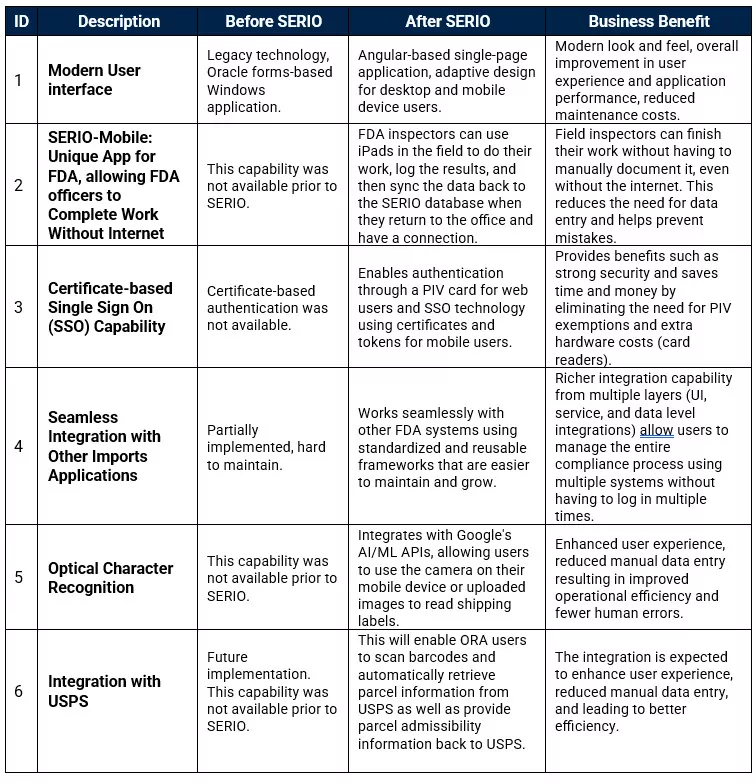
Before and After SERIO:
In conclusion, innovative products like SERIO are vital for fulfilling FDA’s mission in ways outdated legacy systems can’t. Modern applications such as SERIO not only streamline processes but also deliver an improved user experience, making them indispensable tools in enhancing efficiency and ensuring the safety of the public.
The SERIO project team won the 2023 AFCEA InnovateIT Award for the IT Modernization Innovation of the Year.